methyl acetate formation
Isomer-Specific Fuel Destruction Pathways in Rich Flames of Methyl Acetate and Ethyl Formate and Consequences for the Combustion Chemistry of Esters. Preferably the reaction temperature is 160 to 250 C the reaction pressure is 05 to 200 MPa the sample feed space velocity of dimethyl ether is 005 to 3 h 1 and carbon monoxide and dimethyl ether Methyl acetate is produced under conditions where the molar ratio is 20.
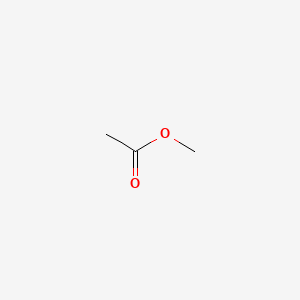
Methyl Acetate Ch3cooch3 Pubchem
This study aims to explore the formation mechanism of ethyl acetate and organic acids in acid rice soup rice-acid soup inoculated with Kluyveromyces marxianus L1-1 through the complementary analysis of transcriptome and proteome.
. C 3 H 7 O 2 Molecular weight. Handling Storage Distribution Hazards Toxicity. It is either used at low doses for chronic illnesses or used concomitantly at high doses during acute flares.
Another method of production is the esterification of methanol and acetic acid in the presence of a strong acid. C 3 H 6 O 2. Melting and Boiling Points.
The molecular or chemical formula of Methyl acetate is C 3 H 6 O 2. Because of its toxicity however benzene can no longer be recommended as a solvent for TLC. Use as a Solvent.
Methyl acetate MeAc esterification process by Eastman Chemical Company Agreda et al 1990. Methyl chloroacetate C3H5ClO2 CID 7295 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. The quantity of K.
Afterward the methoxide inter- mediate attacks a carbonyl group of the anhydride forming methyl acetate and the methylcarbonate ion Figure 2 R3. The net reaction is the formation of acetaldehyde from MeOH CO and H2P4. Empirical formula Hills system for organic substances.
The vapor-phase aldol condensation of methyl acetate MeOAC with formaldehyde HCHO to form methyl acrylate was investigated over solid base catalysts. The Journal of Physical Chemistry A 2007 111 19 4093-4101. Its instability and the formation of artefacts have been subjects of concern in several studies.
Methyl acetate can be further hydrogenated to ethanol which an important chemical and a fuel additive 1. Silica gel is the most widely used sorbent for the essential oils with solvents such as benzene or toluene chloroform methylene chloride ethyl acetate for development. C 3 H 6 O 2.
The melting point of ethyl acetate is -836C while the boiling point is 77C. Copy Sheet of paper on top of another sheet. Methyl acetate MA is produced by various methods such as the esterification of acetic acid the reaction between methanol and acetic anhydride the reaction between peroxycarboxylic acid and acetone and wood pyrolysis.
Copy Sheet of paper on top of another sheet. At 57 C it is a colourless and flammable liquid which is used as a solvent for oils and many resins. To produce methyl acetate methanol is heated alongside acetic acid in the presence of sulfuric acid.
Methyl Acetate Structure Properties And Uses Of C3h6o2 Usual dosing range 10-80 mg IM every 1-2 weeks. The melting point of methyl acetate is -98C while the boiling point is 569C. Switch to calorie-based units.
At a pH of 4 the methyl red indicator turns red it is. Methyl acetate is produced industrially by liquid phase reaction of acetic acid and methanol in presence of an acid catalyst. Methyl formate 109 is converted into AcOH under CO pressure in the presence of Lil and Pd OAc2 95.
Marxianus L1-1 varied significantly in the fermentation process of rice-acid soup and the first and third days were the. 1819 Methylcarbonate cannot serve as methyl. Sulfuric acid is a common catalyst also used in this reaction.
It is a colourless volatile liquid with a pleasant odour and fleeting fruity like the taste. The eluent tolueneethyl acetate 937 is suitable for the analysis and comparison of all of the important essential oils. The Pd- catalyzed reductive carbonylation of methyl acetate with CO and H2 affords acetaldehyde.
Methyl acetate is moderately toxic. It is formed from the condensation of acetic acid and methanol. Information on this page.
A variety of solid base catalysts were prepared by loading Cs 2 O over SiO 2 or Al 2 O 3 as the support materials. Siirola 1996 is a classic application of process intensification PI where the conventional MeAc production process consisting of one reactor and nine distillation columns was replaced by a single RD column. Ethyl acetate is less toxic than Methyl acetate.
What Is Reaction Of Methanol And Ethanoic Acid Quora

Difference Between Methyl Acetate And Ethyl Acetate Compare The Difference Between Similar Terms

Chemical Derivatization Of Acetate Using Methyl Chloroformate Mcf Download Scientific Diagram
Synthesis Of Phenylmethoxycarbonylamino Methyl Acetate Via Ester Formation From Carboxylic Acid Chemsink
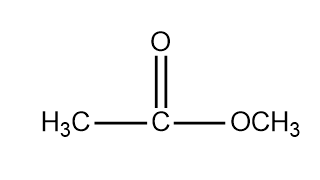
Methyl Ethanoate Is An Ester Write Its Structural Class 11 Chemistry Cbse

Reactions Considered In The Formation Of Methyl Acetate By Alkylation Download Scientific Diagram

Chemical Derivatization Of Acetate Using Methyl Chloroformate Mcf Download Scientific Diagram

Reactions Considered In The Formation Of Methyl Acetate By Alkylation Download Scientific Diagram

Methyl Acetate Metac 99 5 Solvents Wacker Chemie Ag

Datei Synthesis Of Methyl Acetate Svg Wikipedia

Fatty Acid Methyl Ester Production Via Ferric Sulfate Catalyzed Interesterification Sciencedirect

Methyl Acetate An Overview Sciencedirect Topics
What Smell Can I Get When Mixing Methanol With Acetic Ethanoic Acid Quora
Ethyl Acetate Molecule Of The Month March 2003 Html Version
Ethyl Acetate Molecule Of The Month March 2003 Html Version

Methyl Acetate An Overview Sciencedirect Topics

Mass Spectrum Of Methyl Ethanoate C3h6o2 Ch3cooch3 Fragmentation Pattern Of M Z M E Ions For Analysis
File Synthesis Of Methyl Acetate Svg Wikimedia Commons

0 Response to "methyl acetate formation"
Post a Comment